CUET-UG | Chemistry QBank
Hello Aspirants !!!
ScholarWorm welcomes all of his Aspirants who are dreaming to achieve their desired career goal in life.
Here we present you the Questions from the subject of Chemistry for your CUET (Common University Entrance Test). Keep Solving questions to prepare better and improve your scores.
Instructions for Question Paper :-
. The Test Paper is AI generated.
2.The AI bot creates a set of 20 Questions from the pool of questions that is being updated regularly by our expert faculties.
3. Only 1 SET is available per User per Day in free Question Banks. Come Back tomorrow for New SET.
Note :- Number of Questions and Timer is not customizable in Free QBanks.
#1. Incorrect match is :-
#2. The minimum distance between an octahedral and tetrahedral void in fcc lattice will be :-
#3. A liquid freezes at 300K and boils at 400K. If Kf and Kb values for the liquid are 5 and 2.5 °C/molal respectively, then the ratio of latent heat of vaporisation to latent heat of fusion is :-
#4. The enthalpy of formation of CH4(g), H2O(l) and CO2(g) are respectively –74.8 KJ mol–¹, –285.5 KJ mol–¹ and –393.5 KJ mol–¹. Then, the standard enthalpy of combustion of CH4(g) is
#5. Which is the incorrect order of bond angle :-
#6. Which of the following orders of atomic radii are correct :- (a) Li < Be < Na (b) Ni < Cu V > Cr (d) Ti > Zr -~ Hf Correct answer is :-
#7. Dettol is an example of :-
#8. In presence of a strong oxidising agent As2S3 is oxidised to arsinate and sulphate. What is the n-factor of As2S3 .
#9. The shape of IF4+ will be :-
#10. What will be the value of molality for an aqueous solution of 10% w/W NaOH ? (At. Mass Na = 23, O =16, H = 1) :-
#11. In isoelectronic species, which of the following is correct :
#12. In which of the following antiseptic is used for eyes
#13. Carbon-carbon bond formation is possible in the reaction
#14. Which of the following statement is not correct :-
#15. Hall-Heroult method is used during extraction of :-
#16. In hydrogen atom, energy of first excited state is –3.4 eV. Then find out KE of same orbit of hydrogen atom
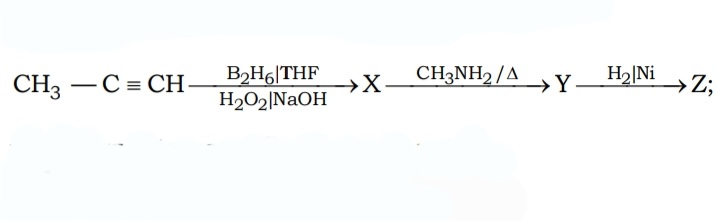
#17. Find the structure of the final product Z :-
#18. Which of the following conditions will increase the voltage of the cell represented by the equation: Cu + 2Ag+ ----> Cu²+ + 2Ag
#19. A substance which gives dark red flame and breaks down on heating to give oxygen and a brown gas is :-
#20. During which of the following extraction of metal poling process is used :-
Finish



