NEET-UG I Chemistry QBank
Welcome Aspirants !!!
ScholarWorm welcomes our aspirants who are always hungry for more knowledge. We are here with our Free Chemistry QBank for NEET-UG to help our students to practice regularly and perform at best of their ability in their exams.
Instruction to Create Question Set :-
1. The Test Paper is AI generated.
2. On clicking the button below the AI bot creates a set of 20 Questions from the pool of questions that is being updated regularly by our expert faculties.
3. A Timer is set for each Test Set, once time is up the AI will automatically submit the test.
4. New Question Set can be created by going backwards and repeating the above steps.
Note :- Number of Questions and Timer is not customizable in Free QBanks.
#1. 44 g of a sample of a compound on complete combustion gives 88 g CO2 and 36 g of H2O. The molecular formula of the compound may be :-
#2. If the sparingly soluble salts M2X, QY2 and PZ3 have the same solubilities, their Ksp values are related as : (S<<1)
#3. As we proceed across the period in periodic table, we find there is a decrease in :-
#4. Which of the following species is not a pseudo halide :-
#5. Which of the following halides has the highest melting point –
#6. Adding powdered Cu and Zn to a solution containing 1.0 M is each of Cu²+ and Zn²+ ions would result into the formation of :-
#7. When copper is heated with conc. HNO it produces ?
#8. The minimum distance between an octahedral and tetrahedral void in fcc lattice will be :-
#9. In HVZ reaction the acidic strength continuously
#10. Which is the incorrect order of bond angle :-
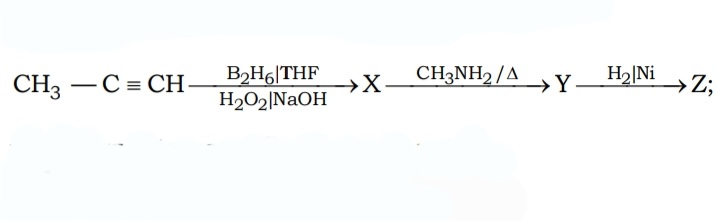
#11. Find the structure of the final product Z :-
#12. In which of the following antiseptic is used for eyes
#13. The electronic configuration of calcium ion (Ca²+ ) is :-
#14. H2O + CH3COOH ----> H3O+ + CH3COO- Which of the following pair act as acids ?
#15. The shape of IF4+ will be :-
#16. The internal energy of an ideal gas increases during an isothermal process when the gas is :-
#17. In which of the following conditions, the reaction will be always spontaneous ?
#18. Which has maximum number of atoms?
#19. Find the pH of a solution when 0.01 M HCl and 0.1 M NaOH are mixed in equal volumes
#20. Correct order of volatility is:-
Finish



